Podcast: Play in new window | Download | Embed
Subscribe: Apple Podcasts | Spotify | Amazon Music | Android | RSS
The topic of low-dose atropine, not FDA-approved, has become one of the most important conversations in pediatric eye care this year, especially as clinicians turn to the STAR Study for clarity on atropine’s role in modern myopia control. Despite decades of global research supporting low-dose atropine and its widespread off-label use in the United States, the FDA’s request for additional data prompted many to re-examine the evidence and understand what the STAR Study truly means for clinical practice today. This discussion occurs alongside significant progress in the field, including the introduction of FDA-approved myopia-control glasses and the continued success of FDA-approved contact lenses, which shape how eye care professionals manage progressive myopia in children.

Table of Contents
What “Low-Dose Atropine Not FDA Approved” Really Means for Clinicians
While the phrase “low-dose atropine not FDA approved” may sound discouraging to parents, clinicians understand its true meaning: atropine remains one of the most researched, trusted, and globally used therapies for slowing myopia progression. Low-dose atropine has been used safely and effectively off-label for years. Its regulatory status reflects a request for additional study details—not a lack of efficacy or safety concerns. Clinicians across the United States continue to prescribe it confidently, grounded in decades of strong international evidence.
FDA-Approved Tools: Glasses, Contacts & the Continued Role of Ortho-K
One of the biggest milestones this year was the introduction of FDA-approved myopia-control glasses. This breakthrough expands access to treatment for children who may not be ready for contact lenses. These glasses join FDA-approved myopia control contact lenses, which have been available longer and are widely used in practices across the country. Meanwhile, orthokeratology (ortho-k) remains a foundational tool in myopia care, even though it is not FDA-approved for myopia control. Dr. Ashley Wallace-Tucker shared that ortho-k is the modality that first sparked her interest in treating progressive myopia and remains a mainstay in her large Houston practice. Together, these options allow doctors to tailor treatment to each child’s needs, lifestyle, and comfort.
The Research Foundation: ATOM, LAMP & LAMP-II
The STAR Study entered a scientific landscape shaped by three foundational studies frequently referenced by Dr. Lyerly.
ATOM (Atropine for the Treatment of Myopia) demonstrated atropine’s ability to slow axial elongation and refractive progression, highlighted dose-dependent response, and established atropine as a credible therapy.
LAMP (Low-Concentration Atropine for Myopia Progression) again showed clear dose-dependent effectiveness across 0.05%, 0.025%, and 0.01% concentrations, shaping practical dosing strategies.
LAMP-II provided multi-year continuation data, confirming sustained benefits, long-term tolerance, and reinforcing atropine’s role in multi-year myopia management.
Inside the STAR Study
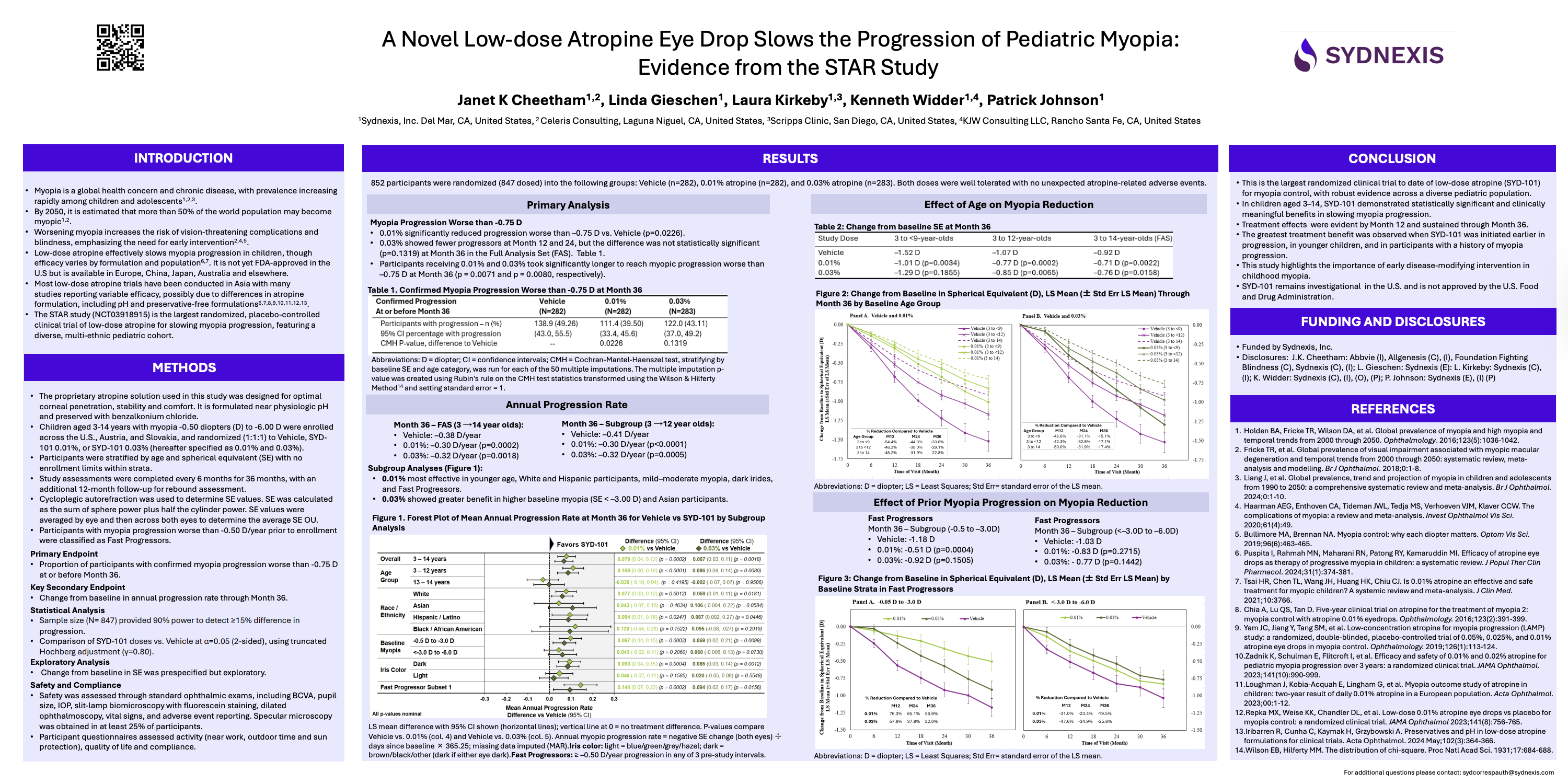
The STAR Study is the largest and most ethnically diverse atropine study to date, evaluating 0.01% and 0.03% atropine versus placebo in more than 800 children across three countries. Key findings showed both concentrations reduced myopia progression, with an average reduction of about 21%. Notably, 0.03% did not outperform 0.01%, differing from ATOM and LAMP. Axial length data was not published, leaving important questions unanswered for clinicians who rely on biometric outcomes. Nonetheless, the study confirmed atropine’s effectiveness.
Why the STAR Study Results Surprised the Myopia-Control Community
Several aspects diverged from expectations. First, STAR showed no clear dose-dependent pattern in the overall population, which differs from the consistent findings in ATOM and LAMP. Second, only children of Asian descent demonstrated the expected dose-dependent behavior, highlighting the importance of diverse populations in clinical research. Third, the omission of axial length data raised questions about study design rather than atropine’s performance.
How Clinicians Should Approach Atropine Today
All three clinicians emphasized that atropine remains a powerful, evidence-based tool despite its current regulatory status. Dr. Wallace-Tucker often begins treatment at 0.05%, a concentration supported by strong research and successful real-world outcomes. Follow-up includes a 1-month tolerance check and, when available, biannual monitoring of refraction and axial length. Both clinicians agreed that biometry is helpful but should not prevent a practitioner from treating a child who is progressing.
Practical Protocols From the Experts
Dr. Wallace-Tucker’s protocol includes identifying appropriate candidates, training parents in drop instillation, completing a 1-month tolerance visit, and monitoring every six months.
Dr. Lyerly’s approach emphasizes presenting all modalities—glasses, contacts, ortho-k, atropine, and lifestyle changes—and empowering families through shared decision-making.
The Bigger Picture: Individualized Myopia Control
A central theme across the discussion was the importance of personalization. With FDA-approved glasses, FDA-approved contacts, ortho-k, lifestyle changes, and atropine, clinicians have more tools than ever to tailor treatment to each child’s unique needs. As Dr. Wallace-Tucker noted, “Whatever you’re doing, individualize it for the person sitting in your chair.”
The STAR Study represents an important milestone in understanding atropine’s role in pediatric myopia. While low-dose atropine, not FDA approved, remains the current regulatory status, decades of evidence and clinical success affirm its continued value. When combined with FDA-approved glasses, FDA-approved contact lenses, ortho-k, and lifestyle strategies, atropine remains a cornerstone in individualized myopia control. Clinicians can confidently continue to use atropine while further data develops.



